The Agile Compliance System for Jira in life science companies.
Ensure compliance with 21 CFR Part 11, Part 820, and IEC 62304 using Jira Cloud as your all-in-one agile ALM solution.
You want a modern solution, but you DON’T want:
The frustration of creating complex configurations and integrations.
The workload of writing system documentation.
The uncertainty of whether your system complies with industry regulations.
You need the Agile Compliance System for Jira.
An all-in-one application lifecycle management (ALM) solution specifically designed for life science companies
using Atlassian Jira Cloud.
The Ultimate Toolbox
Don’t make your users needlessly switch between applications. Use Jira to manage requirements, risks, tests, defects, and backlog items all in one place.
100% Compliant
Confidently deploy Jira, knowing all compliance gaps are closed, and your system complies with 21 CFR Part 11, Part 820, IEC 62304, ISPE GAMP 5, and more.
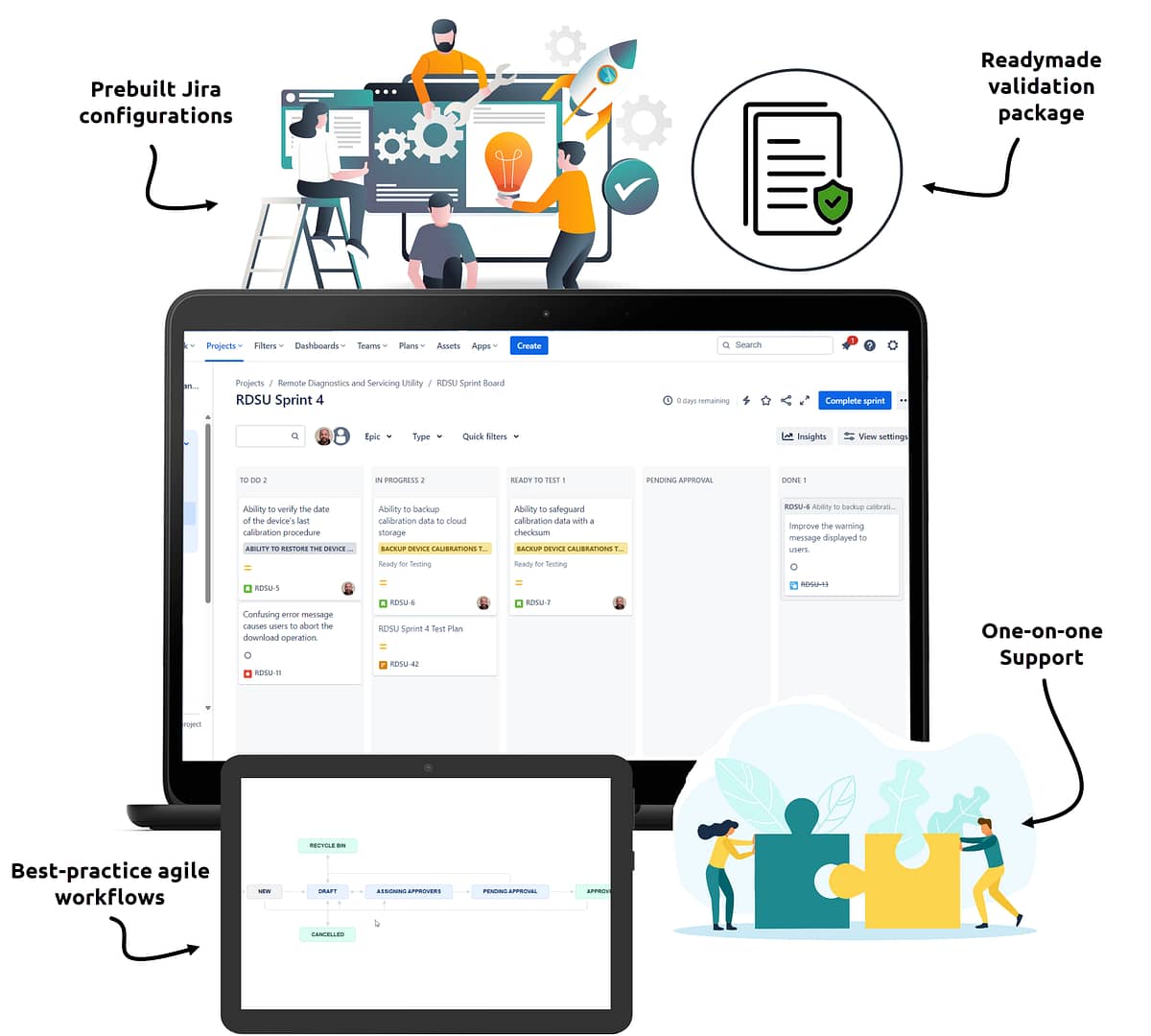
Readymade Configurations
Skip the frustration of designing advanced configurations and integrating third-party apps. You’ll start on the right track with best-practice Jira workflows and configurations already built for you.
Full Traceability
Leverage Jira’s powerful linking capability to establish full traceability across your entire ALM solution, making quality audits a breeze.
Audit-ready Documentation
Avoid weeks of extra work using a prewritten validation package, including a validation plan, user requirements, risk assessments, Part 11 trace matrix, and more…all fully compatible with ISPE GAMP 5.
Specialized Support
Keep your team unstuck with one-on-one support from an expert who understands your industry. Get help with all your questions and challenges, including technical questions about Jira and best practices for using Jira in a highly regulated environment.
I understand the challenges of working in an FDA-regulated environment

Hi! I’m Aaron.
With over 15 years of experience in the life sciences industry, I’ve helped dozens of companies– including Pfizer, Merck, Bayer, and Biogen–to modernize their ALM processes while maintaining regulatory compliance.
I know firsthand the complexities of configuring and validating Jira Cloud for regulatory compliance. It can feel overwhelming, especially when concerned about potential blind spots that could cause problems during audits.
That’s why I’ve created the Agile Compliance System for Jira. It’s designed to transform your uncertainty into confidence, providing pre-built configurations, best-practice agile workflows, ISPE GAMP 5 validation deliverables, and expert support to ensure your Jira Cloud deployment is both compliant and efficient.
Schedule a demo today to find out if the Agile Compliance System for Jira is right for you.
Your path to a compliant ALM solution:
1. Schedule a demo
Schedule a free demo. We’ll discuss your needs, show you how it works, and determine whether the Agile Compliance System for Jira is right for you.
2. Try it risk-free.
I’ll give your team a dedicated trial environment to test Jira as an all-in-one solution. If you don’t like it, you can walk away without paying anything.
3. Launch with confidence.
We’ll work together to quickly configure, validate, and launch Jira Cloud in your company.
How does the Agile Compliance System work?
The Agile Compliance System for Jira is divided into three activities: configuration, validation, and enablement.
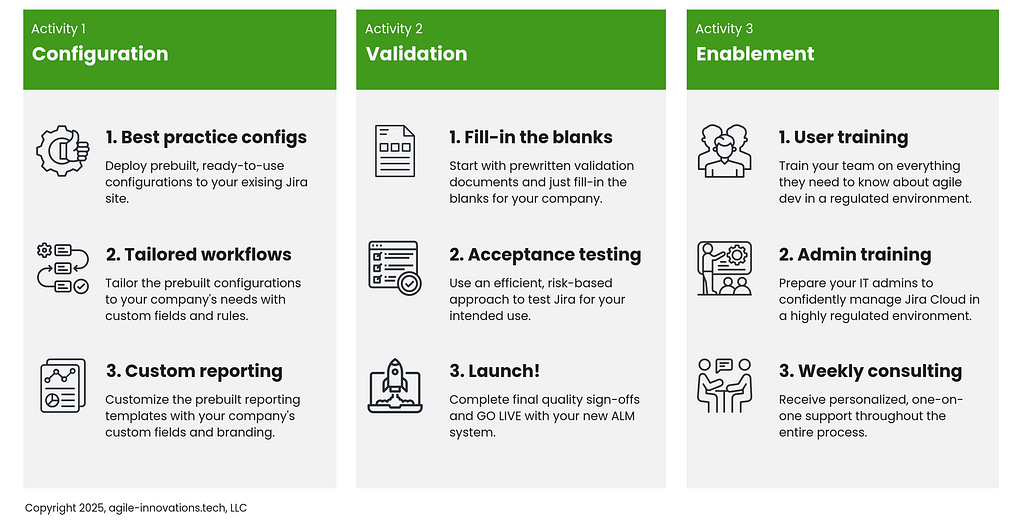
Activity 1: Configuration
Step 1: Start with best practice configurations
First, I’ll deploy prebuilt, ready-to-use configurations to your existing Jira site. These configurations are specifically designed for life science companies so you can experience the benefits of Jira’s best-in-class agility without worrying about compliance gaps.
- Best practice agile workflows: Aligning agile methods with FDA regulations can be confusing and overwhelming. The Agile Compliance System comes with predesigned Jira workflows for requirements, risks, test cases, test plans, test executions, user stories, defects, and more. These workflows are based on modern agile best practices while also complying with 21 CFR Part 11, 21 CFR Part 820, and IEC 62304.
- Part 11 compliant signatures: Avoid the frustration of building a compliant approval process into your Jira workflows. The Agile Compliance System’s workflows include a built-in approval process with configurable approval assignments, Part 11 compliant electronic signatures, and advanced workflow controls to protect approved records from modification.
- The right Jira apps: Don’t waste time sifting through the Atlassian Marketplace trying to find the right apps to plug Jira’s gaps. The Agile Compliance System includes a carefully selected list of third-party apps that seamlessly fit together to form a compliant, all-in-one ALM system. This includes the best apps for requirements management, test management, risk management, reporting, electronic signatures, compliant workflow controls, and more. Better yet, I’ll save you the hassle of figuring out how to wire everything together. Everything is integrated for you as part of the initial deployment.
- End-to-end traceability: The Agile Compliance System contains all the configurations you need to establish full traceability across all your ALM artifacts. From requirements through risks, tests, test evidence, and defects, regulatory reporting will be a breeze.
- Zero blind spots: No need to worry whether your Jira Cloud site is compliant. The Agile Compliance System is based on nearly two decades of experience designing ALM systems for FDA-regulated environments like yours and many years working with Jira specifically. You will be able to confidently deploy Jira, knowing all compliance gaps are tightly closed.
Step 2: Tailor your workflows
Next, we’ll work together to tailor the prebuilt configurations to your company’s specific policies and business rules. This step is about ensuring each process aligns with how your team works and building a system that truly works for you.
- Custom fields and data validation: We’ll add custom fields that capture the exact information your team needs and smart validation rules to bake quality into your processes.
- Personalized user roles: By designing user roles that mirror your real-world team structure, we can streamline your workflow steps to align with your users instead of the other way around.
- Tailored approval processes: We’ll configure your electronic approval rules to align efficient, agile best practices with your company’s policies. As we work, I’ll help guide you in deciding which users would most efficiently provide approval at different levels in the process.
Step 3: Customize the reporting templates
Finally, we’ll update and personalize the prebuilt report templates, eliminating the tedious process of building reports from scratch.
- Eliminate the guesswork: You’ll start with all the templates you need to create audit-ready software releases, including release notes, requirement specifications, test summary reports, requirements traceability reports, and more.
- Tailor to your policies: We’ll update the prebuilt templates with your company’s custom fields and data.
- Reflect your company’s branding: If your company already has prescribed reporting templates, then no problem! We can add branding and boilerplate to the templates or use your own templates directly.
Activity 2: Validation
Step 1: Fill in the blanks
The Agile Compliance System’s validation package provides everything you need to get started with GAMP 5 compliant system validation. From a prewritten validation plan through requirements and risk assessments, just fill in your company’s details and move on. This approach will save you weeks of effort you would otherwise spend writing documents from scratch.
- GAMP 5-compliant Validation Plan: Eliminate guesswork in planning a SaaS-based validation and enjoy quicker quality approvals and smoother audits.
- Validation Deliverables Checklist: Prevent critical validation steps from being missed and avoid potential compliance issues down the line.
- Validation Schedule Template: Manage your validation effort effectively to achieve timely completion of validation tasks and faster time-to-market.
- High-level Risk Assessment: Implement early risk mitigation strategies to reduce the likelihood of compliance failures and enhance overall project success.
- Supplier Risk Assessments: The shared responsibility model of validating SaaS solutions for regulated environments requires that you complete an assessment of each SaaS software vendor. I’ve done the research for you and will provide a detailed supplier assessment for each app used in the Agile Compliance System.
- Part 11 Trace Matrix: Ensure all requirements of 21 CFR Part 11 are satisfied and ensure there are no hidden compliance gaps waiting to be exposed by an auditor.
- User Requirement Specification: Start with a complete requirement specification to fully document and validate the system’s intended use.
- Functional Executable Specification: Use a Gherkin-formatted (BDD) functional specification for a clear and testable understanding of the system’s expected functionality.
- Configuration Specification: Easily understand and maintain your Jira configurations with a detailed configuration specification.
- And more…
Step 2: User acceptance testing
Quickly and compliantly test your configured Jira site to ensure it meets your intended use.
- Prewritten scenarios: Don’t waste time writing tests from scratch. Use prewritten test scenarios that only need to be updated based on your tailored configurations.
- Efficient risk-based testing: It can be confusing to figure out which Jira features actually need to be tested and how much testing they need. My risk-based approach helps you focus on the most critical features based on the risk they add to the system. This means less time spent on check-the-box testing, freeing time for more quality-adding activities.
Step 3: Launch!
Completing validation and releasing the system for general use will be easy if you followed the system this far.
- Final QA approvals: Between the validation package and user acceptance testing results, you’ll have everything you need to get final QA signatures on your Jira site.
- Phased, Smooth Rollout: Launch your new ALM system in a way that minimizes disruption. A carefully planned, phased rollout means your team can adapt gradually and confidently.
Activity 3: Enablement
Step 1: User training
Ensure your rollout is successful with live training sessions for your team:
- Live Training: Agile Compliance in Highly Regulated Environments: Learn the keys to aligning agile practices with regulatory expectations in accordance with AAMI TIR 45.
- Live Training: Agile Requirements Management for Highly Regulated Environments: Learn how to compliantly manage your product requirements inside an agile workflow.
- Live Training: Agile Development for Highly Regulated Environments: Take a deep dive into the best practices for managing development tasks in a highly regulated environment.
- Live Training: Agile Test Management for Highly Regulated Environments: Learn how to to compliantly plan, manage, and summarize your agile testing activities.
- Live Training: Agile Planning for Highly Regulated Environments: Learn how to plan agile software releases and maintain an agile backlog where regulatory compliance is concerned.
- Live Training: Agile Release Management for Highly Regulated Environments: Learn how to leverage Jira to ensure your software releases are compliant and audit-ready for the long term.
- Live Training: Agile Risk Management for Highly Regulated Environments: Learn how to manage your product and system risks directly in Jira and build risk management into your agile workflows.
Step 2: Admin training
For those who manage the system day-to-day, you’ll receive focused training that covers everything from maintaining validated configurations to managing software updates and backups.
- Live Training: Jira Configuration Management: Learn how to maintain your validated system after Go Live through compliant management of Jira configurations.
- Live Training: Jira Workflow Configurations: Learn how to tailor and maintain your new Jira workflows, even if you’ve never configured a Jira workflow before.
- Live Training: Manage Your Vendors: Learn about the shared responsibility model of SaaS validation in a regulated environment where the vendors deploy software updates instead of you. Learn how to monitor for changes, assess impact, and react to problems, including restoring the system from backups.
Step 3: Consulting and support
I want you to be successful. The Agile Compliance System for Jira includes 90 days of personalized support to keep your team unstuck and your deployment on track. This includes weekly coaching sessions, flexible-use consulting hours, and unlimited email-based support.
What if you can’t launch in 90 days? No problem. I’ll work with you for free until you do.
A risk-free commitment to your success.
No up-front payments
When you purchase the Agile Compliance System for Jira, you pay nothing until after I deliver.
Validation guaranteed
If your system is not validated and approved for use within 90 days, I’ll continue working with you for free until it is.
Compliance guaranteed
If my system is ever found to be out of compliance, I’ll refund double your money…contractually guaranteed.
Frequently Asked Questions (FAQs)
How do I know if the Agile Compliance System is for me?
The Agile Compliance System is specifically designed for companies in the life sciences industry (pharmaceutical, medical device, bioengineering, etc.). If you want to use modern agile workflows in a GxP-sensitive environment, this system is designed for you.
Who is it not for?
The Agile Compliance System is designed for companies in highly regulated industries, primarily the life sciences. If you don’t work in a highly regulated industry, then this system will not be a good fit for you. You won’t need the robust controls and reporting that highly regulated companies require.
What’s included when I purchase the Agile Compliance System for Jira?
- Best practice Jira configurations
- Guided configuration tailoring
- Prewritten package of computer system validation (CSV) documents
- Guided system validation
- User training
- Admin training
- Weekly coaching/consulting for 90 days
- Unlimited email support for 90 days
How much does it cost?
I’ll tell you when you schedule a demo.
I know…I hate it when companies say that. 😠It usually means the price is too expensive. But that’s not the case here.
I promise the Agile Compliance System is much less expensive than the competition. It’s definitely less expensive than doing everything yourself.
But I want you to see a demo so you know the system is high quality before you see the low price.
How does the free trial work?
After you see a demo, we’ll discuss your needs and determine if this system will likely be a good fit for you.
If we both feel good, I’ll set your team up with a dedicated Jira Cloud sandbox environment so that you can experiment with the workflows and configurations. We’ll even do some light tailoring to align the configurations with your company’s business rules. (To show you how easily you can configure the system.)
Once you’re confident that the system is 100% compliant and will meet your needs, we’ll move forward with deploying the system to your environment for further configuration and validation.
And if, for any reason, you feel the system won’t meet your needs, then we’ll just delete the trial sandbox, and you won’t have lost anything but your time.
Can I add this to an existing Jira Cloud site?
Yes, the Agile Compliance System configurations can be deployed to an existing Jira Cloud site without impacting any existing configurations.
What if I don’t have an existing Jira Cloud site?
No problem. I can help you create a new one.
How can we validate a cloud (SaaS) application when the vendor (Atlassian) controls the software environment?
Validating a cloud (SaaS) application for a GxP environment follows a shared responsibility model. In addition to testing the cloud software against its intended use, you must also establish whether you trust the SaaS vendor to help maintain the software in a validated state.
That might sound hard, but it’s easier than it sounds. My prewritten validation plan, supplier assessments, and other readymade deliverables will make this process a breeze.
I wrote about this a bit in a blog post: Understanding Part 11 Compliance in Jira Cloud
And of course, we can discuss this further when you schedule a demo.
My company is slow to validate new systems. What happens after the 90 days of support is over?
No problem. I get it. It’s sometimes difficult to control the speed of approval in a life science company.
So, if you don’t complete validation within the initial 90-day support period, I’ll continue working with you for free until you do.
Why? Because I’m confident you’ll be successful, even if things take a little extra time for your organization.
How can I get started?
What will you learn when you schedule a demo?
1. Is this right for you?
I only want happy customers. We’ll start with a brief discussion about your current situation and needs to ensure the Agile Compliance System is right for you.
2. How does it work?
I’ll show you exactly how the process works. You’ll see a live demo of everything you’ll receive in the Agile Compliance System and how it will be delivered.
3. How much does it cost?
Next, we’ll discuss how much the Agile Compliance System for Jira costs. (Less than you expect!) And I’ll show how the cost is almost insignificant compared to the amount of money it will save you.
4. How to get started?
Finally, we’ll discuss the steps to get you set up with your own trial environment so that you can evaluate the Agile Compliance System for yourself.
Is YOUR Jira site truly Part 11 compliant?
Download this FREE traceability matrix to find your compliance gaps.